What are organic and inorganic molecules?
In chemistry, molecules are classified as either organic or inorganic. Organic molecules contain carbon, while inorganic molecules do not. Organic molecules are typically found in living things, while inorganic molecules are found in non-living things.
Organic molecules are composed of a variety of elements, including carbon, hydrogen, oxygen, nitrogen, and sulfur. They are typically large and complex, and they can have a wide range of shapes and sizes. Inorganic molecules, on the other hand, are typically composed of only a few elements, such as hydrogen, oxygen, nitrogen, and chlorine. They are typically small and simple, and they have a regular shape.
Organic molecules are essential for life. They are the building blocks of proteins, carbohydrates, lipids, and nucleic acids. Inorganic molecules are also important for life, but they play a less direct role. They are involved in a variety of processes, such as the transport of oxygen and the regulation of pH.
The study of organic and inorganic molecules is a vast and complex field. However, it is an essential field of study for anyone who wants to understand the world around them.
Organic and Inorganic Molecules
Organic and inorganic molecules are two broad classes of chemical compounds that differ in their composition and properties. Organic molecules contain carbon, while inorganic molecules do not. Organic molecules are typically found in living things, while inorganic molecules are found in non-living things.
- Structure: Organic molecules are typically large and complex, while inorganic molecules are typically small and simple.
- Composition: Organic molecules are composed of a variety of elements, including carbon, hydrogen, oxygen, nitrogen, and sulfur. Inorganic molecules are typically composed of only a few elements, such as hydrogen, oxygen, nitrogen, and chlorine.
- Properties: Organic molecules are typically combustible, while inorganic molecules are typically non-combustible. Organic molecules are also typically insoluble in water, while inorganic molecules are typically soluble in water.
- Reactivity: Organic molecules are typically more reactive than inorganic molecules.
- Biological significance: Organic molecules are essential for life, while inorganic molecules play a less direct role.
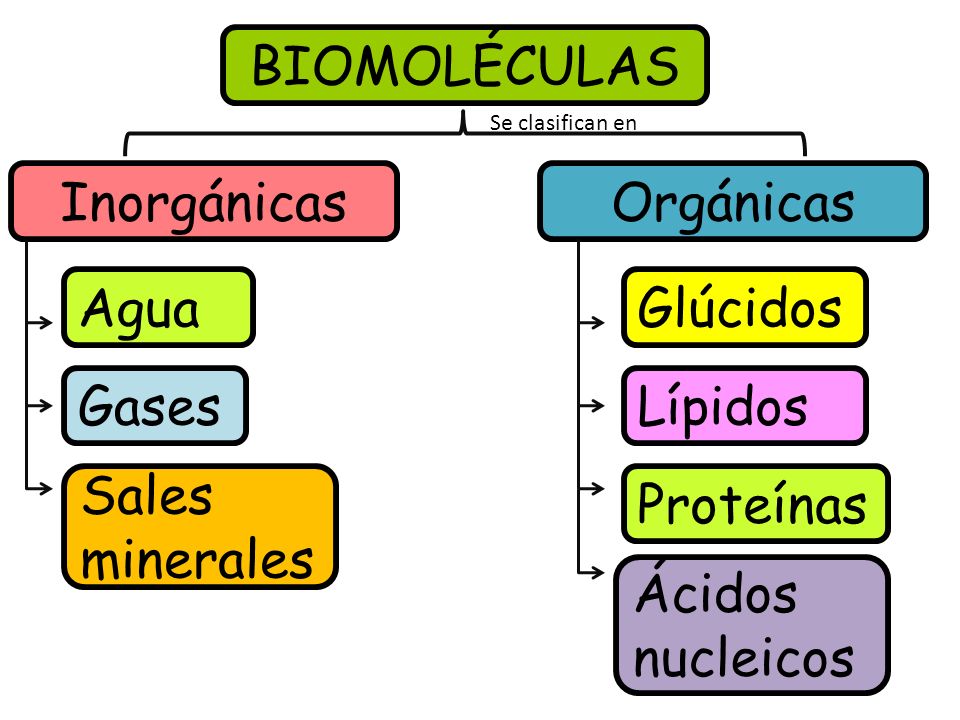
- Examples: Examples of organic molecules include proteins, carbohydrates, lipids, and nucleic acids. Examples of inorganic molecules include water, carbon dioxide, and sodium chloride.
Organic and inorganic molecules are essential to life. They play a variety of roles in the human body, including providing energy, building and repairing tissues, and regulating bodily functions. The study of organic and inorganic molecules is a vast and complex field, but it is an essential field of study for anyone who wants to understand the world around them.
Structure
The structure of organic and inorganic molecules is one of the key factors that distinguishes them from each other. Organic molecules are typically large and complex, while inorganic molecules are typically small and simple. This difference in structure is due to the fact that organic molecules contain carbon, while inorganic molecules do not.
- Size: Organic molecules are typically much larger than inorganic molecules. This is because organic molecules are composed of long chains of carbon atoms, while inorganic molecules are typically composed of only a few atoms.
- Shape: Organic molecules can have a wide variety of shapes, while inorganic molecules are typically more regular in shape. This is because organic molecules are able to form covalent bonds with each other in a variety of ways, while inorganic molecules typically form ionic bonds.
- Complexity: Organic molecules are typically more complex than inorganic molecules. This is because organic molecules contain a variety of different functional groups, while inorganic molecules typically contain only a few different types of atoms.
The difference in structure between organic and inorganic molecules has a significant impact on their properties. Organic molecules are typically more reactive than inorganic molecules, and they are also more likely to form polymers. Inorganic molecules, on the other hand, are typically more stable than organic molecules, and they are less likely to form polymers.
Composition
The composition of organic and inorganic molecules is one of the key factors that distinguishes them from each other. Organic molecules are composed of a variety of elements, including carbon, hydrogen, oxygen, nitrogen, and sulfur. Inorganic molecules are typically composed of only a few elements, such as hydrogen, oxygen, nitrogen, and chlorine.
- Elements present: Organic molecules contain carbon, while inorganic molecules do not. This is the key difference between the two types of molecules.
- Variety of elements: Organic molecules contain a wider variety of elements than inorganic molecules. This is because carbon is able to form covalent bonds with a variety of other elements.
- Complexity: Organic molecules are typically more complex than inorganic molecules. This is because they contain a larger number of atoms and a wider variety of elements.
The composition of organic and inorganic molecules has a significant impact on their properties. Organic molecules are typically more reactive than inorganic molecules, and they are also more likely to form polymers. Inorganic molecules, on the other hand, are typically more stable than organic molecules, and they are less likely to form polymers.
Properties
The properties of organic and inorganic molecules are closely related to their composition and structure. Organic molecules are typically composed of carbon, hydrogen, and oxygen, and they have a high molecular weight. This makes them combustible, meaning that they can burn easily. Inorganic molecules, on the other hand, are typically composed of elements such as sodium, chlorine, and potassium, and they have a low molecular weight. This makes them non-combustible.
- Combustibility: Organic molecules are typically combustible, while inorganic molecules are typically non-combustible. This is because organic molecules contain carbon, which is a highly flammable element. Inorganic molecules, on the other hand, typically contain elements that are not as flammable, such as sodium, chlorine, and potassium.
- Solubility in water: Organic molecules are typically insoluble in water, while inorganic molecules are typically soluble in water. This is because organic molecules are non-polar, while inorganic molecules are polar. Non-polar molecules do not dissolve in water, while polar molecules do.
The properties of organic and inorganic molecules have a significant impact on their behavior in the environment. Organic molecules are typically found in living things, while inorganic molecules are typically found in non-living things. This is because organic molecules are more likely to be broken down by biological processes than inorganic molecules.
Reactivity
The reactivity of organic and inorganic molecules is a key factor that distinguishes them from each other. Organic molecules are typically more reactive than inorganic molecules because they contain carbon. Carbon is a highly reactive element, and it is able to form covalent bonds with a variety of other elements. This makes organic molecules more likely to undergo chemical reactions.
The reactivity of organic molecules is important because it allows them to participate in a wide variety of biological processes. For example, organic molecules are used to build proteins, carbohydrates, lipids, and nucleic acids. These molecules are essential for life, and they could not be synthesized without the reactivity of organic molecules.
The reactivity of organic molecules also has a number of practical applications. For example, organic molecules are used to make plastics, fuels, and pharmaceuticals. These products are essential to modern society, and they could not be made without the reactivity of organic molecules.
The reactivity of organic molecules is a fundamental property that has a significant impact on their behavior in the environment and their use in a wide variety of applications.
Biological significance
In the context of "concepto de moleculas organicas e inorganicas", the biological significance of organic and inorganic molecules is a key aspect that distinguishes them. Organic molecules are essential for life, while inorganic molecules play a less direct role. This is because organic molecules are the building blocks of all living things, while inorganic molecules are typically found in non-living things.
- Building blocks of life: Organic molecules are the building blocks of all living things. They are used to make proteins, carbohydrates, lipids, and nucleic acids. These molecules are essential for life, and they could not be synthesized without the reactivity of organic molecules.
- Energy source: Organic molecules are also an important source of energy for living things. When organic molecules are broken down, they release energy that can be used to power the body's cells.
- Regulation of bodily functions: Inorganic molecules also play an important role in living things, although their role is less direct than that of organic molecules. Inorganic molecules are involved in a variety of bodily functions, such as the regulation of pH, the transport of oxygen, and the transmission of nerve impulses.
The biological significance of organic and inorganic molecules is a complex and fascinating topic. It is a topic that is essential to our understanding of life itself.
Examples
In the context of "concepto de moleculas organicas e inorganicas", the examples provided serve to illustrate the distinct characteristics and roles of these two major classes of molecules. Organic molecules, characterized by their carbon content and complex structures, are essential for life and encompass a wide range of biomolecules such as proteins, carbohydrates, lipids, and nucleic acids. These molecules perform crucial functions in living organisms, from providing structural support and energy to facilitating genetic information transfer.
- Building Blocks of Life: Organic molecules are the fundamental building blocks of all living organisms. Proteins, for instance, are responsible for a vast array of biological functions, including metabolism, cell signaling, and immune response. Carbohydrates provide energy and structural support, while lipids serve as energy reserves and form cell membranes. Nucleic acids, such as DNA and RNA, carry genetic information and play a central role in heredity and protein synthesis.
- Inorganic Molecules in Life Processes: While inorganic molecules may not be directly involved in life's processes, they play essential supporting roles. Water, for example, is a universal solvent and crucial for maintaining cellular homeostasis. Carbon dioxide is involved in photosynthesis, the process by which plants convert sunlight into energy. Sodium chloride, commonly known as table salt, is essential for regulating fluid balance and nerve impulse transmission.
The examples provided in "Examples: Examples of organic molecules include proteins, carbohydrates, lipids, and nucleic acids. Examples of inorganic molecules include water, carbon dioxide, and sodium chloride." thus highlight the diverse nature and significance of organic and inorganic molecules, showcasing their complementary roles in the intricate tapestry of life and non-living matter.
FAQs on the Concepto de Moleculas Organicas e Inorganicas
This section provides answers to frequently asked questions (FAQs) about the concept of organic and inorganic molecules. These questions are designed to address common misconceptions and provide a deeper understanding of the topic.
Question 1: What is the key difference between organic and inorganic molecules?
Answer: The key difference between organic and inorganic molecules lies in the presence or absence of carbon. Organic molecules contain carbon atoms, while inorganic molecules do not.
Question 2: Are all organic molecules living?
Answer: No, not all organic molecules are living. Organic molecules can be found in both living and non-living things. For example, methane (CH4) is an organic molecule that is found in natural gas.
Question 3: Are all inorganic molecules non-living?
Answer: Yes, all inorganic molecules are non-living. Inorganic molecules are typically found in minerals, rocks, and other non-living things.
Question 4: Are organic molecules always more complex than inorganic molecules?
Answer: Yes, organic molecules are generally more complex than inorganic molecules. This is because organic molecules are composed of long chains of carbon atoms, while inorganic molecules are typically composed of simpler structures.
Question 5: Are inorganic molecules always less reactive than organic molecules?
Answer: No, not always. Some inorganic molecules, such as acids and bases, can be highly reactive. However, organic molecules are generally more reactive than inorganic molecules.
Question 6: What are some examples of organic and inorganic molecules?
Answer: Examples of organic molecules include proteins, carbohydrates, lipids, and nucleic acids. Examples of inorganic molecules include water, carbon dioxide, and sodium chloride.
These FAQs provide a concise overview of the key differences between organic and inorganic molecules. By understanding these differences, students can gain a deeper appreciation for the diversity and complexity of the chemical world.
Conclusion
The concept of organic and inorganic molecules is fundamental to our understanding of the chemical world. Organic molecules are the building blocks of life, while inorganic molecules play a vital role in many geological and industrial processes. The distinction between these two types of molecules is based on their composition and structure. Organic molecules contain carbon, while inorganic molecules do not. Organic molecules are typically more complex and larger than inorganic molecules. They are also more likely to be combustible and insoluble in water.
The study of organic and inorganic molecules is a vast and complex field. However, it is an essential field of study for anyone who wants to understand the world around them. Organic and inorganic molecules are essential to life, and they play a vital role in many industrial and geological processes. By understanding the difference between these two types of molecules, we can better understand the world around us.
2020 Ultimate Guide: Unlocking The Potential Of Keyword Optimization
The Shocking Truth: Did Sigurd Assassinate A Physician?
Essential IRS Forms 940: Download And Instructions

Qué son las biomoléculas y cómo se clasifican Educación Activa
Química y Algo Más! Las Biomoléculas y el ADN

BIOMOLÉCULAS